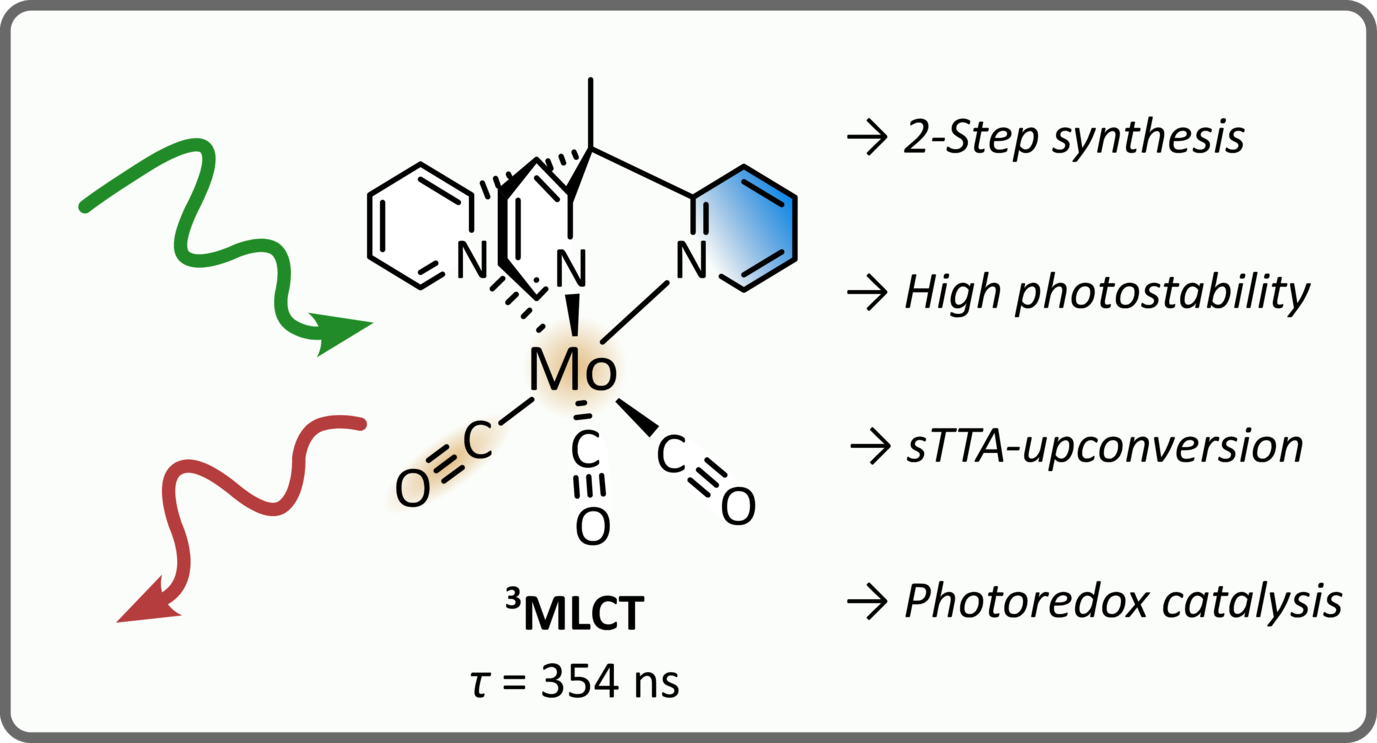
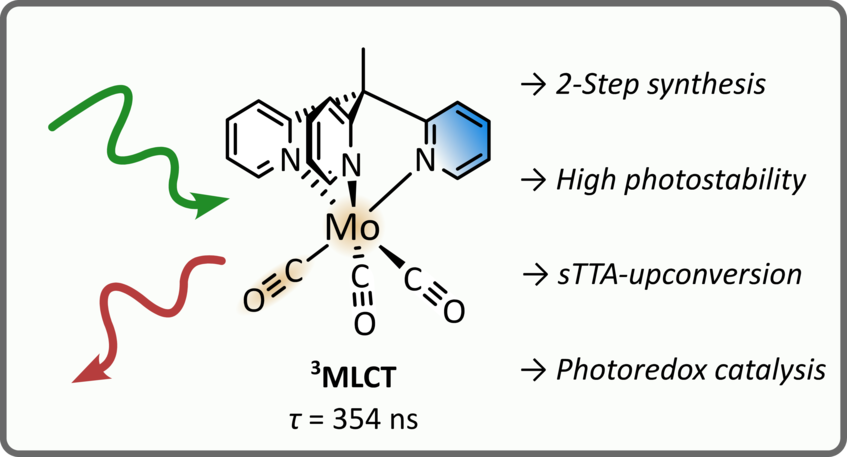
Stable Molybdenum(0) Carbonyl Complex for Upconversion and Photoredox Catalysis
Photoactive complexes with earth-abundant metals have attracted increasing interest in the recent years fueled by the promise of sustainable photochemistry. However, sophisticated ligands with complicated syntheses are oftentimes required to enable photoactivity with nonprecious metals. Here, we combine a cheap metal with simple ligands to easily access a photoactive complex. Specifically, we synthesize the molybdenum(0) carbonyl complex Mo(CO)3(tpe) featuring the tripodal ligand 1,1,1-tris(pyrid-2-yl)ethane (tpe) in two steps with a high overall yield. The complex shows intense deep-red phosphorescence with excited state lifetimes of several hundred nanoseconds. Time-resolved infrared spectroscopy and laser flash photolysis reveal a triplet metal-to-ligand charge-transfer (3MLCT) state as the lowest excited state. Temperature-dependent luminescence complemented by density functional theory (DFT) calculations suggest thermal deactivation of the 3MLCT state via higher lying metal-centered states in analogy to the well-known photophysics of [Ru(bpy)3]2+. Importantly, we found that the title compound is very photostable due to the lack of labilized Mo–CO bonds (as caused by trans-coordinated CO) in the facial configuration of the ligands. Finally, we show the versatility of the molybdenum(0) complex in two applications: (1) green-to-blue photon upconversion via a triplet–triplet annihilation mechanism and (2) photoredox catalysis for a green-light-driven dehalogenation reaction. Overall, our results establish tripodal carbonyl complexes as a promising design strategy to access stable photoactive complexes of nonprecious metals avoiding tedious multistep syntheses.