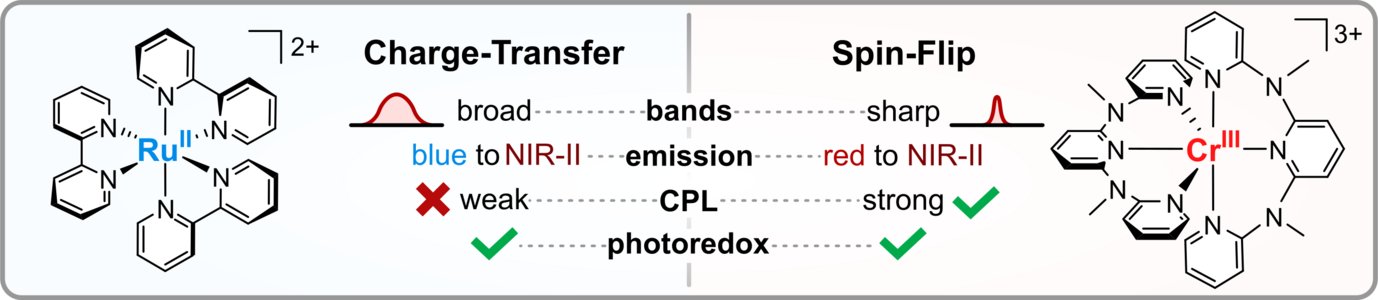
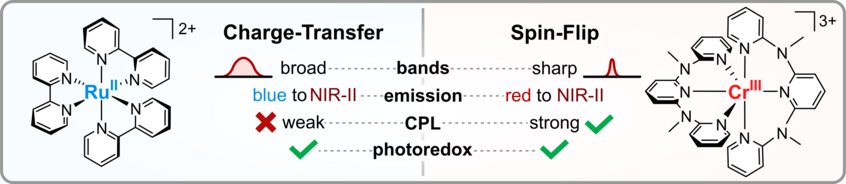
Charge-Transfer and Spin-Flip States: Thriving as Complements
Photoactive organometallic compounds are fuelling advances in fields like photocatalysis, solar energy conversion and display technology. Most of these systems like the well-known [Ru(bpy)3]2+ rely on charge-transfer excited states that have been in the focus of research for decades. In a charge-transfer transition an electron is excited e.g. from a metal d-orbital to a pi* orbital of the ligand (MLCT). Their most common deactivation pathway is via metal-centered states that are distorted and non-luminescent. In recent years, the discovery of a new type of high-performing photoactive chromium(III) complexes – the Molecular Rubies – reignited interest in a special kind of metal-centered states, the so-called spin-flip states. These states only differ from the electronic ground state by a flipped electron spin. Hence, the bonding situation does not change, which leads to narrow emission bands and high photostability. This review delineates similarities and unique features of (luminescent) charge-transfer and spin-flip states, covering fundamentals and applications like photocatalysis, sensing and circularly polarized luminescence in a tutorial approach.