Solids Go Bio: Inorganic Nanoparticles as Enzyme Mimics
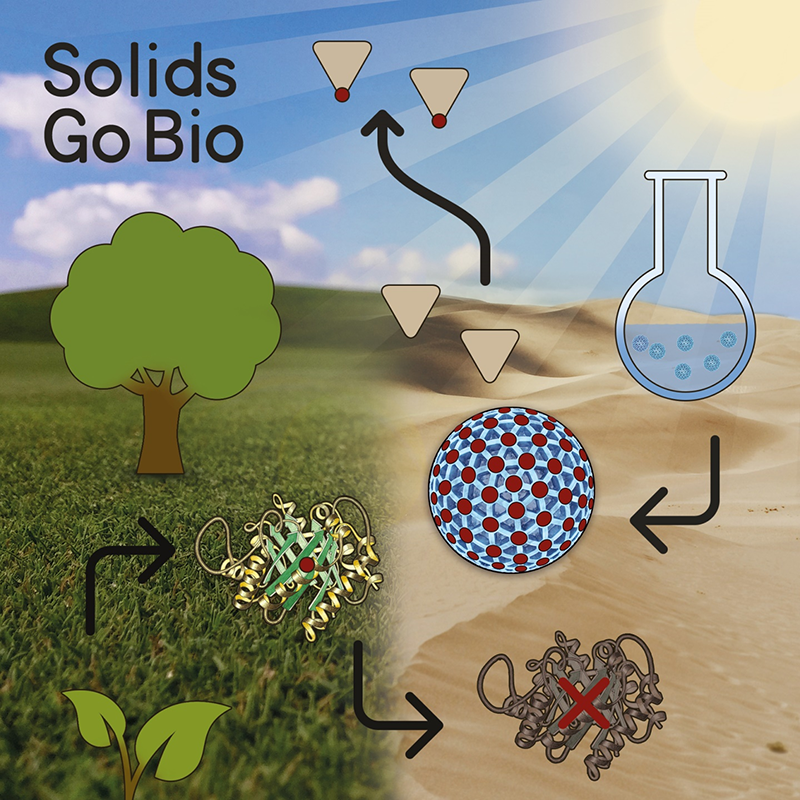
Enzymes belong to the most selective and efficient catalysts known to men and have evolved to almost perfection over the last billions of years. So the question arises why natural enzymes have not reached broad industrial utilization. The answer is pretty simple in this case, as the production of natural enzymes is associated with immense costs and they generally suffer from an overall low stability and shelf-life, especially when exposed to external stimuli such as heat, sun light, and change of pH or solvent. Enzymes generally work unrivalled in terms of efficiency and specifity in their natural environment (aqueous buffers, pH around 7, ≈37°C), but quickly get misfolded or start denaturating when used in organic solvents or with temperatures of above 60°C (most industrial applications).
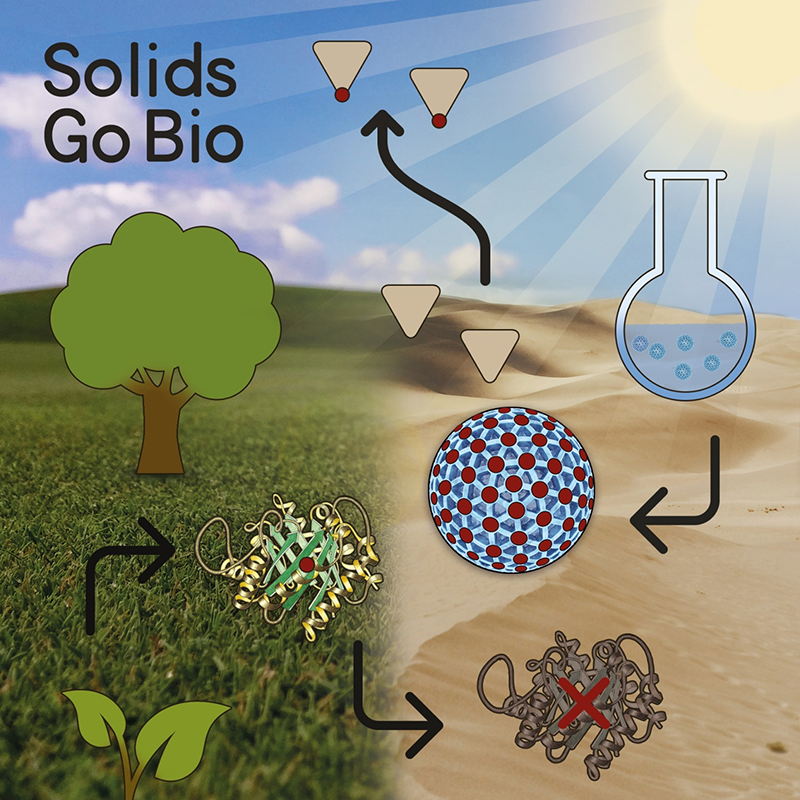
Inorganic nanoparticles on the other hand are readily available by cost-efficient synthesis and have been used for many years as heterogeneous catalysts in organic solvents. The past three decades, have seen a wide variety of materials, including metal complexes, polymers, and other biomolecules, that mimic the structure and function of naturally occurring enzymes. Among these, inorganic nanomaterials bear huge potential, because they are more stable than their natural counterparts, while having large surface areas and sizes comparable to those of natural enzymes. Therefore, an enormous amount of inorganic nanomaterials with intrinsic enzymatic activities has been reported. The microreview highlights the recent progress in the field of “Inorganic Nanoparticles as Enzyme Mimics” and focuses on enzymatic systems that only recently have been made available.