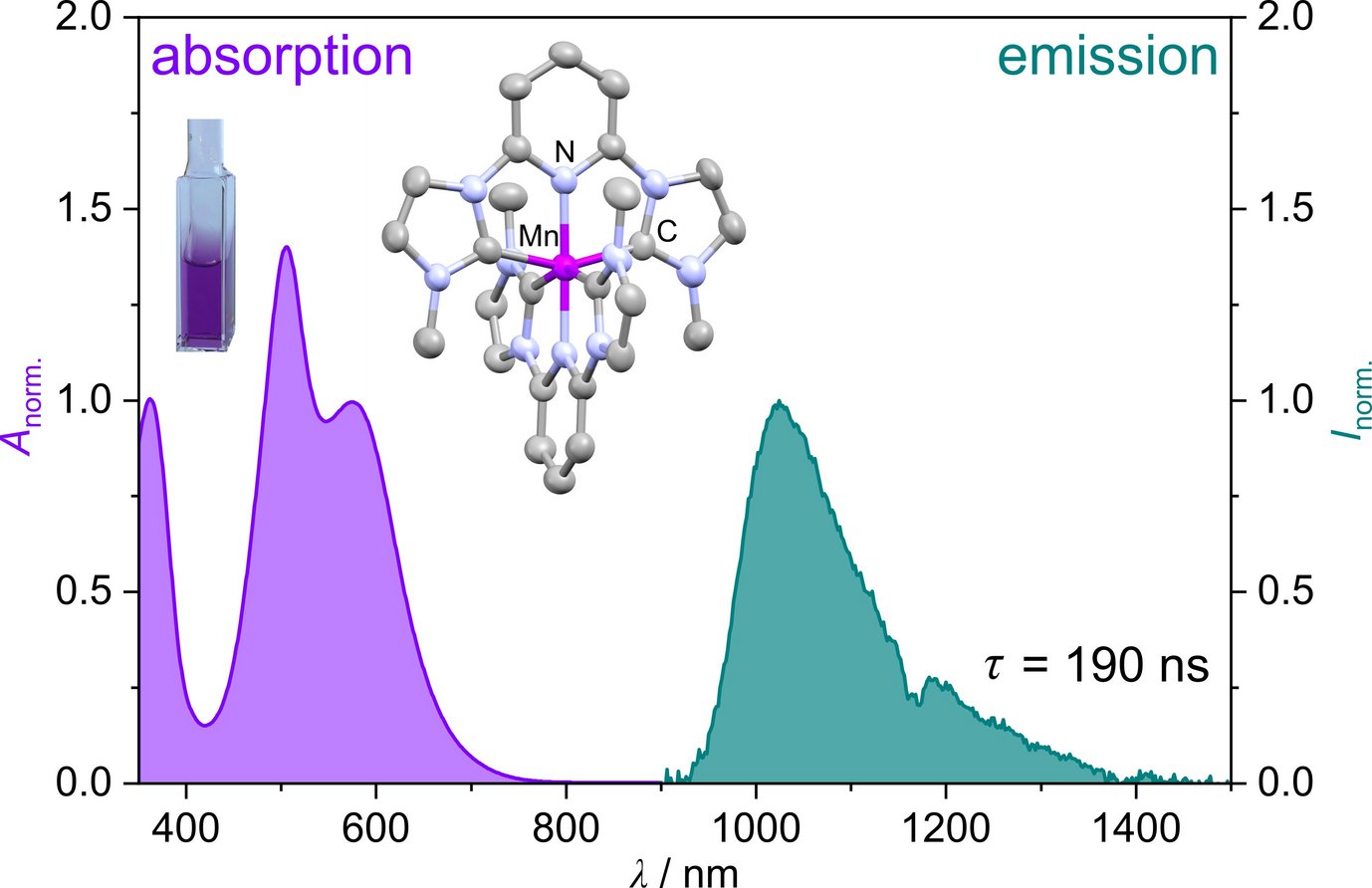
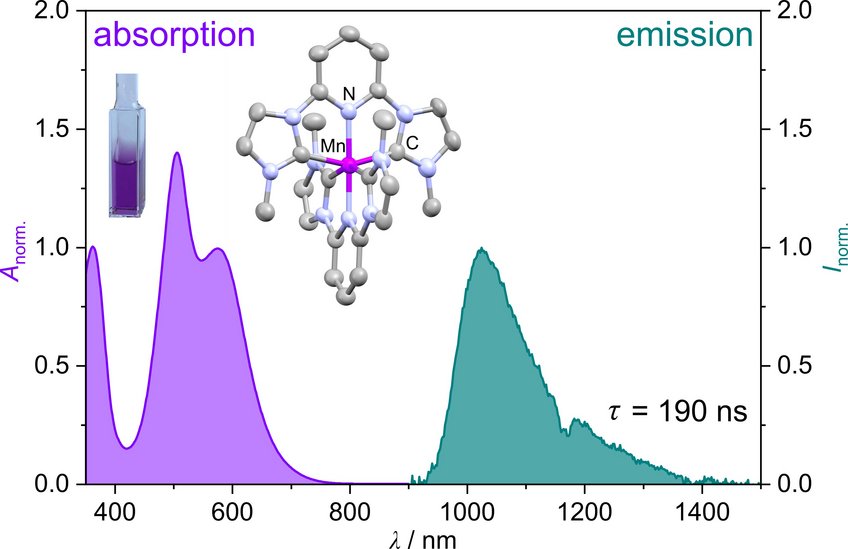
A manganese(I) complex with a 190 ns metal-to-ligand charge transfer lifetime
In recent decades, photochemistry has emerged as a transformative approach to drive chemical reactions with exceptional precision. Unlike thermal methods, which rely on the random distribution of energy among molecules, photochemical excitation allows selective access to specific, high-energy electronic states, opening reaction pathways that are often inaccessible via heat. This unique control makes photochemistry crucial for applications such as photocatalysis, molecular sensing, and solar energy conversion. Traditionally, these applications have relied on precious metal complexes based on ruthenium, iridium and osmium, valued for their strong luminescence and long-lived excited states. However, these metals are scarce, expensive, and environmentally taxing. Manganese, in contrast, is over 100.000 times more abundant than ruthenium, but its application in photochemistry has been limited by two longstanding challenges: short excited-state lifetimes and synthetically complex multistep procedures, often requiring 9 to 10 steps.
Together with their coworkers Professor Katja Heinze and PhD student Sandra Kronenberger, who are associated to the Sustainable Photochemistry Focus Group of the MPGC, recently reported a novel manganese(I) complex that addresses both challenges. The complex can be synthesized in a single step directly from commercially available starting materials, making it highly accessible. Remarkably, the complex exhibits emission with a lifetime of 190 nanoseconds, a value on par with those of complexes based on rare and expensive noble metals. This discovery positions manganese(I) as a promising alternative for photophysical and photocatalytic applications, offering both economic and environmental advantages.